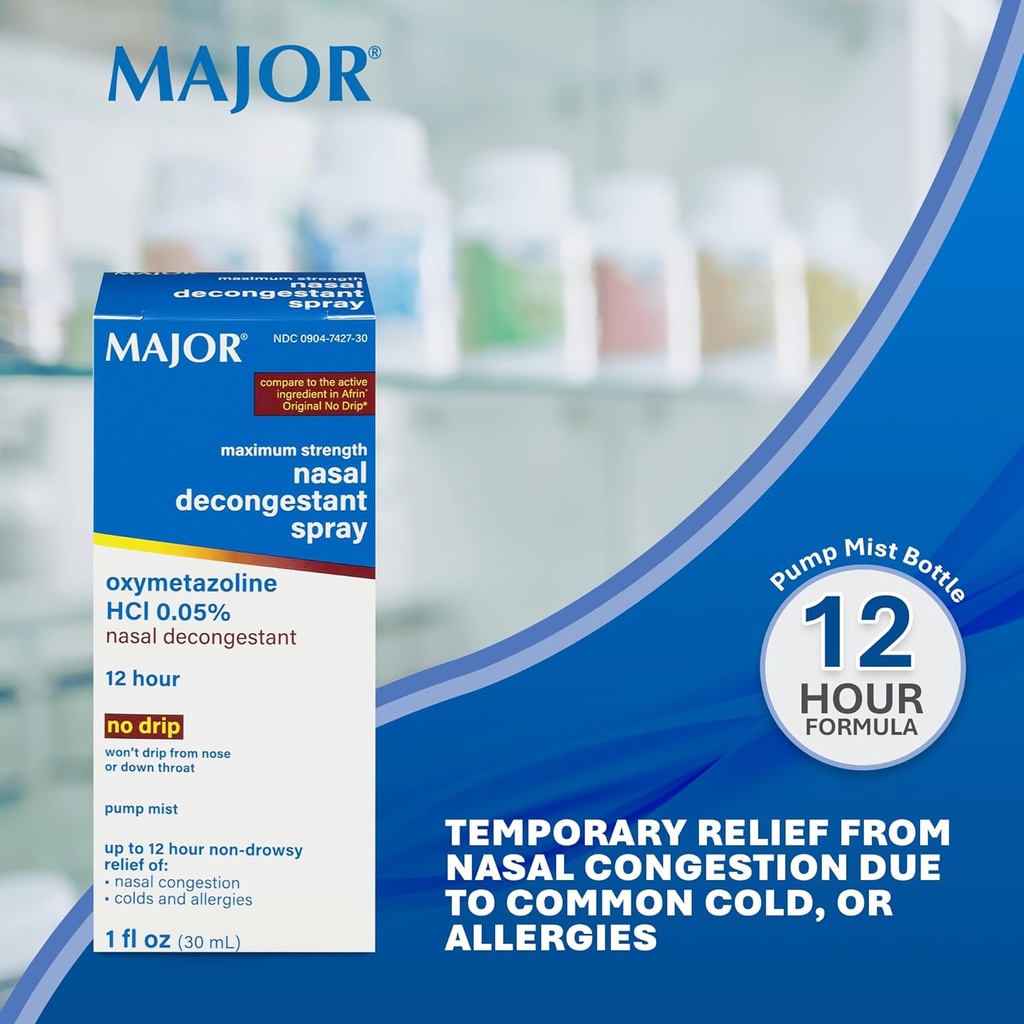
MAJOR Maximum Strength Nasal Decongestant Spray, Oxymetazoline HCl 0.05% Pump Mist, Non-Drowsy Nasal Spray, up to 12-Hour Relief from Nasal Congestion, Colds, and Allergies 1 Fl. Oz. (6-Pack)
3 sold in last 24 hours
- NASAL DECONGESTANT SPRAY – Contains 0.05% Oxymetazoline HCl to temporarily relieve nasal congestion from the common cold, hay fever, or upper respiratory allergies. Provides up to 12 hours of relief with a convenient nasal spray pump mist.
- HELPS RELIEVE SINUS PRESSURE —This nasal spray decongestant is formulated to temporarily reduce sinus pressure and swollen nasal membranes. Suitable for adults and children ages 6 to under 12, with adult supervision.
- NON-DROWSY, NO DRIP NASAL SPRAY – Quality non-drowsy, no-drip formula that won’t run from the nose or down the throat. Designed for comfortable use to alleviate nasal congestion, cold, and allergy symptoms.
- HOW TO USE - Shake well before use. 2–3 sprays per nostril every 10–12 hours. Do not exceed 2 doses in 24 hours or for more than 3 days. Children under 6: Ask a doctor.
- MAJOR PHARMACEUTICALS - At Major Pharmaceuticals, we believe wellness should be accessible to everyone. We pride ourselves on our legacy of delivering affordable, high-quality OTC and vitamin supplements, trusted by healthcare professionals and pharmacies across 50+ therapeutic categories.
Tags |
Important information
Ingredients
Active Ingredient: Oxymetazoline hydrochloride 0.05%. Inactive Ingredients: Benzalkonium Chloride Solution, Benzyl Alcohol, Dibasic Sodium, Edetate Disodium, Microcrystalline Cellulose abd Carboxymethylcellulose Sodium, Monobasic Sodium Phosphate, Polyethylene Glycol, Providone, Purified Water.
Legal Disclaimer
Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition.
Package Dimensions
13.7 x 10.71 x 3.03 inches; 11.68 ounces
| Product Benefits | Sinus Control or Nasal Decongestant |
| Ingredients | Purified Water. or Polyethylene Glycol or Benzyl or Dibasic Sodium or Edetate Disodium or Monobasic Sodium Phosphate or Providone or Active Ingredient: Oxymetazoline Hydrochloride 0.05%. Inactive Ingredients: Benzalkonium Chloride Solution or Microcrystalline Cellulose Abd Carboxymethylcellulose Sodium |
| Expiry Date | Dec 2027 |
| Number Of Items | 6 |
| Specific Uses For Product | Nasal Decongestion |
| Active Ingredients | Oxymetazoline Hydrochloride 0.05% |
| Product Type | Medication |
| Brand | Major |

?unique=45c9327)




?unique=45c9327)